
About The Preeclampsia Registry
Last Updated on May 16, 2023
Launched in 2013, the Preeclampsia Registry was built to create a partnership between preeclampsia survivors and researchers to advance our understanding of preeclampsia. As participants share their experiences, approved researchers are provided with access to “de-identified” health information – information that does not contain participant names or contact information. This helps researchers to find patterns that can lead to findings and further studies. The registry also accelerates studies as researchers are able to gather cohorts for new studies or pose new questions.
The Preeclampsia Registry also gives its participants the option of learning about other research studies for which they may qualify and provides them with the means of connecting with researchers.
Most importantly, patient registries unite the patient voice with research. Patients have questions, theories, and interests that are often different from investigators conducting formal research. By engaging patients in the research process, as we can through patient registries, the chances for discovery and improvement are an even greater possibility.
Have questions? Contact Research Manager VeeAnn Argyle at registry@preeclampsia.org.
Preeclampsia Registry Advisory Council
The Preeclampsia Registry Advisory Council (PRAC) played a key role in the development of the Preeclampsia Registry and continues to be essential to its integrity. The PRAC provides representation amongst many disciplines including epidemiology, internal medicine, pediatrics, nephrology, genomics, obstetrics and gynecology (including Maternal Fetal Medicine), industry, National Institutes of Health, Centers for Disease Control, patient/consumer, and the Preeclampsia Foundation Medical Medical Advisory Board.
Jim Roberts, MD
Co-Chair
University of Pittsburgh
Magee Women's Research Institute
Sarosh Rana, MD, MPH
Co-Chair
University of Chicago Medicine
Eleni Tsigas, BA
Preeclampsia Foundation, Chief Executive Officer
Principal Investigator, Preeclampsia Registry
Arun Jeyabalan, MD
University of Pittsburgh
Michelle Owens, MD
University of Mississippi
Janet Rich-Edwards, ScD
Harvard Medical School
Ellen Seely, MD
Brigham and Women's Hospital
Ananth Karumanchi, MD
Howard Hughes Research Institute
Harvard Medical School
Terry Morgan, MD, PhD
Oregon Health Sciences University
Alison Roberts, CNM
West Berkeley Family Practice
Kent Thornburg, PhD
Oregon Health and Science University
Tom Easterling, MD
University of Washington
Kyu-Ho Lee, MD PhD
Medical University of South Carolina
Robert Powers, PhD
University of Pittsburgh
Donna Russell, MHA
Precia Group
Kenneth Ward, MD
Juneau Biosciences
VeeAnn Argyle
Preeclampsia Foundation, Registry Manager

I had a routine prenatal appointment at 27 weeks, where my blood pressure was slightly elevated, the midwife I had seen that day was pretty c...
ReadMoreResearchers, please contact our Research Manager if you would like the Preeclampsia Foundation to help you recruit participants for a research study or focus group, or have any questions about the Preeclampsia Registry.
Related Articles

Your story is needed to improve outcomes for moms like you. Add your voice to critical preeclampsia research to ensure that every story is heard.

Frequently asked questions about the Preeclampsia Registry, a patient-driven registry and biobank.

The Preeclampsia Foundation offers research funding, study recruitment, and other patient engagement services to researchers.
We provide research grant funding to advance progress towards detection, prevention, or treatment of preeclampsia, HELLP syndrome, and other hypertensive disorders of pregnancy.

Preeclampsia is a life-threatening hypertensive disorder, affecting 2%–5% of pregnancies, that remains poorly understood. In a recent study published by Physiological Genomics that was...
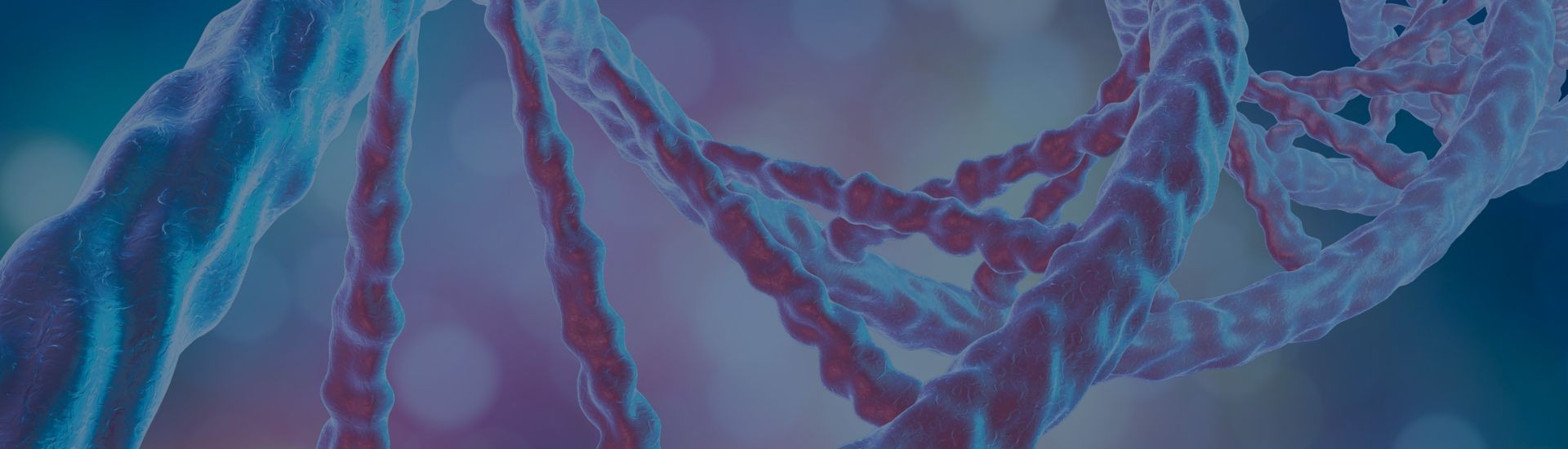
Echocardiography, commonly referred to as cardiac ultrasound or an “echo”, generates visual images of the heart known as echocardiograms. This imaging technique is especially informative d...
There is an increased cardiovascular risk after a pregnant woman has preeclampsia. Previous research found that there are signs of early cardiovascular aging when a woman is six months postpartum...

The accuracy of medical history is critical for the care of any patient. Preeclampsia increases the risk of complications in future pregnancies and is also associated with an increased risk of cardiov...
The authors of this paper note that an increasing number of people are being readmitted to the hospital within six weeks of discharge home after delivery and that many of these persons had a hypertens...



